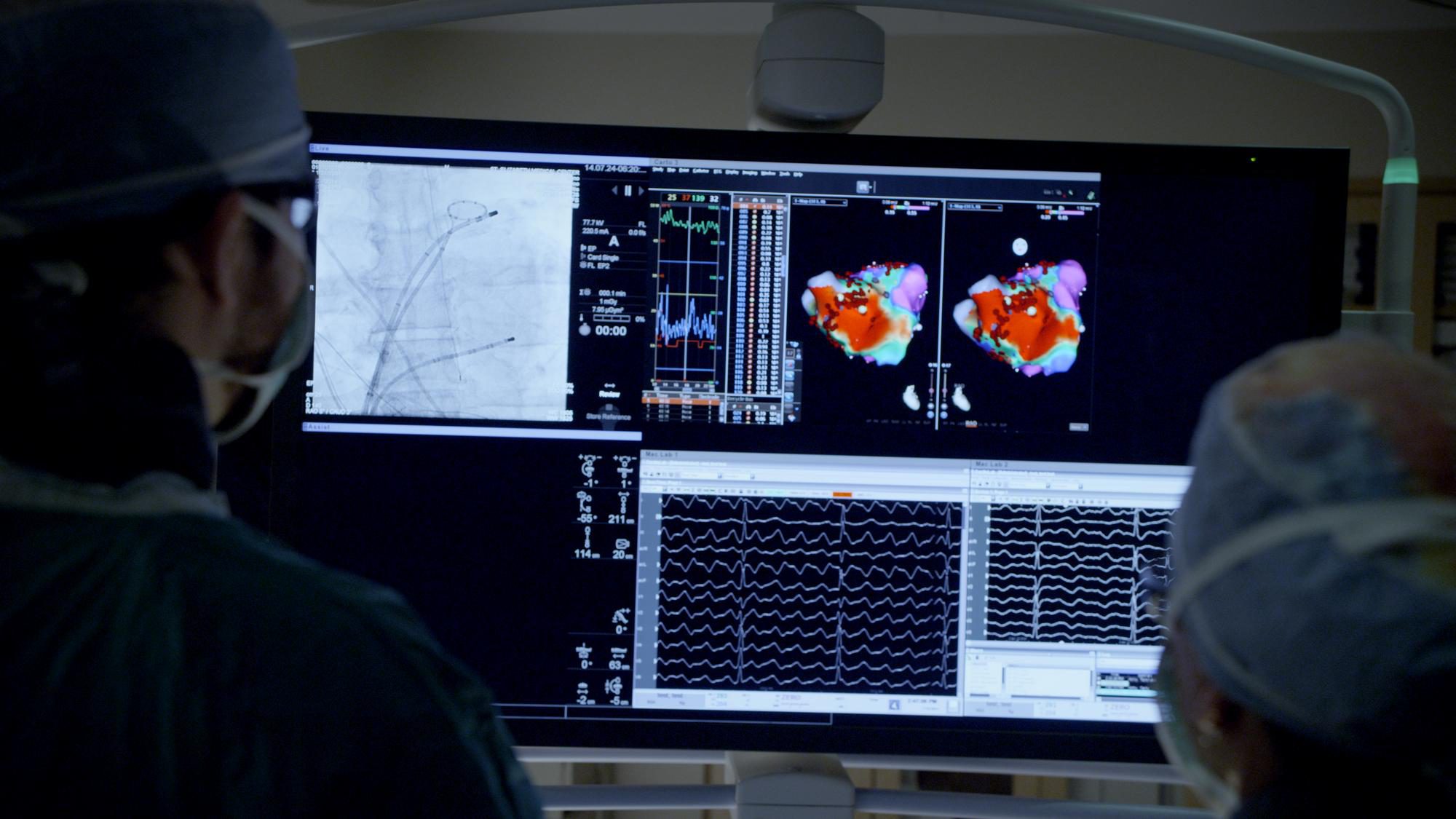
Leadless Cardiac Pacemaker Technology
Pacemakers monitor your heart and provide electrical stimulation to manage slow or irregular heartbeats. A conventional pacemaker requires traditional surgery to place the device in your chest, along with insulated wires (leads) between the device and your heart. This requires a large incision and a visible lump where the device is implanted in your chest.
Leadless cardiac pacemakers are:
- Much smaller (only a tenth the size).
- Only require a small incision.
- Placed directly in your heart (no lump).
To implant the device, doctors make a small incision in your upper leg and thread a catheter (small, flexible tube) up through a blood vessel and into your heart. The device is fully retrievable, so it can be repositioned during the implant procedure and removed later if necessary.
Schedule an Appointment
Call: (859) 331-3353
Benefits Over Traditional Pacemakers
Leadless pacemakers:
Florence Wormald Heart & Vascular Institute at St. Elizabeth was the first facility in Greater Cincinnati to offer these advanced cardiac pacemaker technologies: